
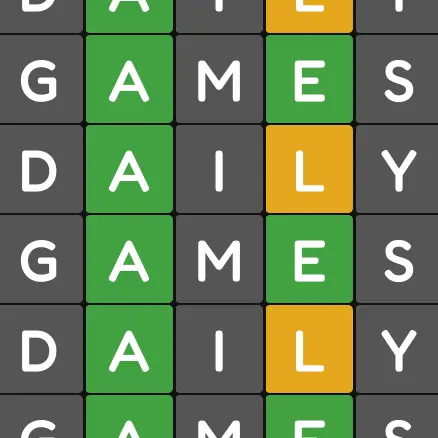




[Bracket City]
September 8, 2025
https://www.theatlantic.com/games/bracket-city/
Rank: 👑 (Kingmaker)
❌ Wrong guesses: 0
Total Score: 100.0
🟩🟩🟩🟩🟩🟩🟩🟩🟩🟩


🙂 Daily Quordle 1323
6️⃣4️⃣
5️⃣3️⃣
m-w.com/games/quordle/
🟨⬜🟨🟩⬜ 🟨⬜⬜⬜⬜
⬜🟨⬜⬜⬜ ⬜⬜⬜🟩⬜
⬜⬜⬜⬜⬜ ⬜🟩⬜🟨⬜
⬜⬜🟩⬜⬜ 🟩🟩🟩🟩🟩
⬜🟨⬜🟨⬜ ⬛⬛⬛⬛⬛
🟩🟩🟩🟩🟩 ⬛⬛⬛⬛⬛
⬜⬜⬜🟨⬜ ⬜⬜⬜⬜⬜
⬜⬜⬜🟨⬜ ⬜⬜⬜⬜🟨
⬜🟨⬜⬜⬜ 🟩🟩🟩🟩🟩
🟩🟨⬜🟨⬜ ⬛⬛⬛⬛⬛
🟩🟩🟩🟩🟩 ⬛⬛⬛⬛⬛


Daily Akari 😊 Sun Aug 31, 2025 ✅Solved in 1:17✅ https://dailyakari.com/


Wordle 1,530 2/6
🟩🟩⬛🟩🟩
🟩🟩🟩🟩🟩
Exquisite luck


Is this calculated by assuming the wavefunction is static?
Typically sorta? The way the Schrödinger equation is typically solved is by taking linear combinations of eigenfunctions (of the Hamiltonian) and making them time-dependent with a time-dependent phase out front.
The eigenfunctions are otherwise time-independent since you can usually make the Hamiltonian be time independent.
If the problem is easier to think about with a time-dependent Hamiltonian, you can use the Heisenberg formulation of quantum mechanics, which makes the wavefunctions static and lets the operators evolve in time. This can be helpful in a number of situations—typically involving light.
Like, maybe a steady-state eigenfunction of the system’s evolution with an eigenvalue that’s 1, or another root of unity.
I assume you mean eigenfunction of the Hamiltonian here, but the eigenvalue associated with that eigenfunction would be the energy of the state, so you can’t really make it be a root of unity (it must, in fact, be fully real since energy is an observable)


I’m basically a tourist in quantum physics with no more than approximate understanding of several concepts, and I don’t think I’ll ever fully understand a field that took dozens of Nobel prize winners and multiple lifetimes to formulate
That’s everyone, honestly. Physics is big enough these days that I don’t think anyone could get all of it.
I always thought of “position” as simply a point in Euclidean space described by a vector, but I’m guessing that doesn’t translate directly to quantum mechanics because the uncertainty principle gets introduced with having to account for momentum.
That very much still is the case (though it’s technically Minkowski space once you introduce special relativity); when you measure the position of an electron, you will get a single point as far as we can tell. It’s just that there is a range of locations you might see it in when you observe it.
Does that mean that two electrons can, at the instant they are observed, have no meaningful distance between them, only different momentum?
Hmm… yes?
I believe that two electrons ‘occupy the same space’ (down to some uncertainty) when they scatter off of each other. As stated above, they are point-like, though, so you would need infinite precision to make them properly overlap.
But there is a less finicky way to do it:
If you observe position (down to some accuracy), you can’t observe momentum (down to a related accuracy)—that is the core of the uncertainty principle. That being said, if you have perfect knowledge of their momentum, you will have no knowledge of their position, which will allow them to be ‘in the same place’ insofar as they both are everywhere all at once.
This can actually be done practically by cooling them down: if you constrain their temperature/energy/momentum, you can get them to ‘overlap’ through uncertainty. When this happens, they actually pair up, adding their one-half spins up to either 0 or 1. This integer spin makes the pair a boson and allows them to occupy the same states as other pairs (note that the electrons themselves cannot occupy each others’ states, but the pairs can, and these ‘Cooper pairs’ become the principle particles of interest). This lets them (the pairs) flow through each other without scattering, which is how superconductors work.


I think my answer here does a disservice to your last two questions, but they’re pretty interesting, so I’d like to try answering them here. That being said, I believe a proper explanation would require a greater understanding of quantum field than I currently have.
Regardless, let’s start with
How many properties does it take to describe, for example, an electron?
Wavefunctions are typically described using just two parameters: position and time. That being said, the specifics of a theory will often add two more ‘parameters:’ spin and charge.
For every position in space and time, there are four possible electrons:
Negatively charged with up spin,
Negatively charged with down spin,
Positively charged with up spin, and
Positively charged with down spin
Now, there are a couple conservation laws that prevent the charge of an electron from changing, so we split these four into two categories based on charge, and call members of the latter (positively charged) category ‘positrons.’
There are no such conservation laws preventing an electron from changing its spin, however, so it’s not worth calling the up and down spin electrons separate particles;1 we instead keep track of an electron’s up-ness and down-ness by sticking a two-component vector into the wavefunction.
So, to clarify, you should only need to know the electron’s position and spin at a given time to know its state.
Or, rather, knowing these will give you the local value of the wavefunction of the electron, which is defined at all points in space, at all points in time, and for both possible spins.
This wavefunction describes the state that the particle is in, and it is the solution to our Schrödinger equation.
The magic of quantum mechanics is that there are much fewer possible wavefunctions than you might expect for a given system.2
In many cases, we can actually label each of the allowed wavefunctions with one (or a handful of) quantum number(s),3 which will be integers. The energy (n) and angular momentum (j) are common quantum numbers.
So we can describe states with quantum numbers while the actual wavefunctions associated with these states describe the electrons that occupy them via spin, position, and time.
This means that when our Pauli exclusion principle requires different wavefunctions for different electrons, it is also enforcing that they occupy different states i.e. have different quantum numbers.
But then how does spin let you have two electrons in the same state? It doesn’t
If two electrons have ‘different spins,’ it actually means they have different wavefunctions (remember! Wave-funcs are functions of spin) and are thus in different states. In the absence of a magnetic field, however, up-spin and down-spin are effectively identical in all other aspects, so you will often see that which is described as one state is actually two.
Now for your other question:
What kind of precision does it take to tell whether the two states are identical?
None!
States are quantized, meaning they are described by a (typically finite) set of integer-valued quantum numbers, and if any of the qn’s are different, so too are the states.
It is worth noting that two different quantum states will have zero expected positional overlap, so you can say that they are in different places even if both wavefunctions are defined everywhere.
That being said, the mathematically-enforced inability of electrons to occupy the same state will certainly look like an equal and opposite to any force that energetically favors an already-occupied state… which is why you can pick up your pencil.
Now, if you start to apply a whole lot of pressure on the system, you start to change the states that solve the Schrödinger equation in the first place. So your neutron star isn’t infinitely stable.
1 spin can also mix while charge can’t, so an electron can be partially up and partially down, but not partially negative and partially positive. This is really just a consequence of the (in)ability to switch between states, but I figured I would mention this for clarity.
2 if you let some infinities be ‘fewer than others
3 technically, any linear combination of states can constitute an allowed wavefunction (this is superposition), but, this will constrain the set of allowable wavefunctions for other electrons, meaning the total number of allowed wavefunctions remains the same.


ELI5: Imagine a ring on a table. They putting some marbles in that ring. They can roll anywhere, right? Well, quantum mechanics makes you put those marbles in a neat little grid. The marble can still ‘roll’ like a piece moves on a chess board: to unoccupied spots around it. If there’s something already in it, though, it won’t be able to move there. Each of those spots is a ‘quantum state.’
(Importantly, the ring on the table typically represents spots in phase space—something that involves both position and momentum—rather than real space)
ELI15: A quantum state is a state that a quantum system can be in. That isn’t very helpful, but you are asking about something very fundamental and thus something very difficult to describe without getting into the math.
It is important to note that a ‘state’ means very little without knowing what ‘system’ it describes. A system is made up of all the interacting particles that pull on each other and is what we are interested in describing.
All of these interactions collectively generate the ‘potential’ at every point in space and time, and knowing this potential allows us to write down the specific Schrödinger equation that we want to solve. This equation will have a limited1 number of solutions.
Consider your computer science class-instance again. Each instance of the class represents a state, but there is a larger class called System that has an array-type property that holds the States that solve it. Additionally, you should add another property to the State class: occupation. For bosons, this property is an Int, but for fermions, it is a Bool.2 Now, in order for a boson to join the system, it needs to increment the occupation counter on one of the States, but for a fermion to join, it needs to find a State with a False occupation and flip it to True.
Allowing for multiple fermions in the same state would result in a loss of information; nothing changes if a True state is flipped to True. This is thus not allowed, forming the basis of the Pauli exclusion principle.
ELI25: A quantum state is a linear combination of eigenstates for an observable quantum operator—typically the Hamiltonian of a system. Saying that the PEP disallows fermions from entering the same quantum state is a crude way of saying that their combined wavefunction must be antisymmetric:
Ψ(𝛙1, 𝛙2) = -Ψ(𝛙2, 𝛙1)
But then if
𝛙1 = 𝛙2
We get
Ψ(𝛙1, 𝛙2) = -Ψ(𝛙1, 𝛙2)
Which means
Ψ = 0
And is thus disallowed
1 limited means ‘quantized’ here—not ‘finite’
2 notably, you get a different system and thus a different set of States for every particle type. So a clump of neutrons would be unable to ‘exclude’ an electron

A couple notes:
First, renormalizarion was hand-wavy when it was first introduced, but it has since been made mathematically rigorous. Additionally, renormalization is a mathematical process to make a theory self-consistent. If you consider it an odd idea because it is physically nonsense, I would caution against forming a physical intuition from any given accurate mathematical model. Especially with fundamental quantum mechanics—there’s a reason why there are several interpretations of QM and have been for a century.
Second, and arguably more importantly: this ScienceDaily article is extremely misleading. The original paper (linked by OP in another comment) says
This is a scenario where the inflaton does not exist, and thus opens up the possibility to provide a picture of inflation that is model independent
So the paper does rid itself of the inflaton field, which is, as you said, a bit of a hand-wave. Crucially, however, it does not abandon inflation—in fact, it explains those “for no reason”s that you mentioned.


Angling the holster so that it’s already pointing at the target when it clears the waist
4-Chan is a self-hate group. It’s full of people using slurs to refer to themselves, so I’m not sure you can really call this a non-cruel context


a) it’s quite common, so a lot of people have encountered it
b) feet are rather dirty, so it evokes disgust in those that don’t have it
This creates an environment in which people can commiserate in their disgust with others by means of insult. This creates a meme that people will take on as a belief to join the in-group / avoid being targeted by the majority.
It’s the same as a lot of arbitrary hate, honestly.


Dopamine does a lot of things. It does not cross the blood-brain barrier.
So taking this would be all side effects and no benefits


It is conceivable that the glue was put there for another purpose, and the pigeons simply landed in it. That’s just not likely in this case


Disclaimer: I have no experience with this drug class, and my medical knowledge is that of a novice.
“Don’t expose it to light” typically means “don’t leave it in the sun too long.”
These warnings usually mean that the chemical will denature / break down when exposed to light or heat which leads to a decrease in efficacy. Most chemicals will only do this in response to IR or UV light. If the reaction is from IR, then the primary worry is keeping it cool enough. By the nature of indoor lights, your bathroom light is almost certainly not emitting enough UV to cause a reaction.
If the drug is colored (I don’t believe this to be the case here), then visible light could cause a reaction, though I would still find it unlikely that a drug would lose sufficient efficacy due to a bathroom light.
I wouldn’t worry unless it got too warm for too long.
I can’t say anything about the label turning red without a bit more info about the container itself (is it just a vial?), but it sounds like it might be an indicator that you took your last dose.
As always, contact your doctor or provider if you have concerns.


And now we have a nice, comma-separated array of strings for wherever that might be useful ;)